Improvement in attention with DYANAVEL XR
Tablet vs placebo2
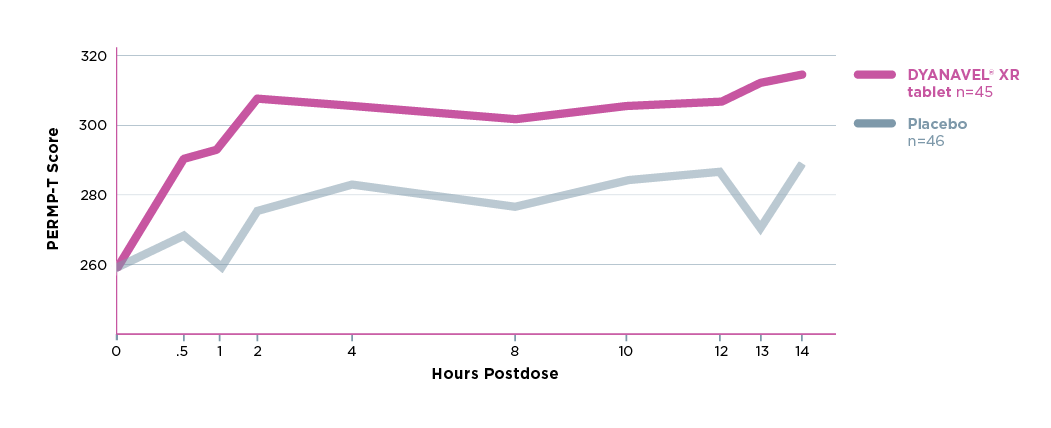
Study Design: This study employed a 5-week forced dose–titration phase. Eligible subjects aged 18 to 60 years who met DSM-5 criteria for ADHD were randomized to double-blind DYANAVEL XR Tablet or matching placebo, taken orally once daily beginning the day after the baseline visit. Subjects were titrated from a 5mg starting dose up by 5-mg increments each week until a final dose of 20mg for 14 ± 3 days prior to visit 5. At Visit 5, efficacy assessments included the administration of serial PERMPs predose and at 0.5, 1, 2, 4, 8, 10, 12, 13, and 14 hours postdose. Safety and tolerability were assessed at each study visit, including direct questioning about sleep, appetite, mood, and psychotic adverse events.2
Safety Information from Clinical Study: In the forced-dose, double-blind phase 3 study conducted in adults, no serious adverse events were reported. In the study the most commonly reported treatment-emergent adverse events (TEAEs) (>5%) in the DYANAVEL XR Tablet group and greater than placebo were: Decreased appetite, Insomnia, Dry mouth, Irritability, Headache, Dizziness, Initial insomnia, Nausea, Tachycardia, Anxiety.
DYANAVEL XR was shown to improve ADHD symptoms compared to placebo when measured with a standardized performance test, the PERMP‡, a that measures the ability to initiate a task, self-monitor/stay on task, and complete written seatwork.
The primary efficacy endpoint—the mean PERMP-T score across all postdose time points at Visit 5 (last visit)—was statistically significantly higher in the DYANAVEL XR tablet group than in the placebo group (P = 0.0043)
See how DYANAVEL XR tablet provides continuous medication release4
‡ The PERMP is a validated, time-sensitive, skill-adjusted test consisting of math problems to be completed at multiple time points (administration of serial PERMPs). It is a robust, objective measure of the ability to initiate a task, self-monitor/stay on task, and complete written seatwork. The PERMP does not test for mathematical ability or the ability to learn math because the difficulty of problems is adjusted to the existing math skill level of each participant.5
PERMP-T, Permanent Product Measure of Performance Total.






